Written by: Adam Tzur
Last updated: 22.01.2018
Acknowledgements: Greg Nuckols of StrongerByScience
The information presented here is not medical advice. Please consult your doctor before making lifestyle changes.

Plain Language Summary
Sarcopenia
We lose muscle mass and strength as we age. This is known as sarcopenia. Muscle loss could begin in our 30s, if we are sedentary. Genetics and lifestyle play a major factor and it varies from individual to individual.
Why we become weaker
There are several reasons why we become weaker: Our nervous system becomes more inefficient, our muscle quality decreases, our anabolic hormone secretion decreases, our bodies develop chronic inflammation and anabolic resistance.
Preventing age-related muscle loss
- Eat more protein, at least 1.2g/kg BW up to 2.2 g+/kg
- Do strength training with high intensities and volumes
- Have an active lifestyle outside the gym
- Supplement various nutrients like vitamin E, D, and omega-3s (if you are deficient)
Introduction
Aging is a complicated phenomena, but most of us have seen what happens to the human body as it ages. Naturally, we start to wonder what will happen to our own bodies. People have different experiences and opinions online. Some people suggest that aging kills the body's hypertrophic potential, while others say age has no effect on gains until we get well into our 70s. This article examines different aspects of aging and how it affects our body composition and athletic potential.
If you find this article difficult to read or understand, just scroll down to or ctrl+f this section: How to treat and prevent age-related muscle loss (practical applications for your life).
Sarcopenia
The body slowly loses its ability to build muscle mass and increase strength as we age (Horstman et al., 2012; Timmons & Gallagher, 2016; Brook et al., 2016; McLeod et al., 2016; Mitchell et al., 2016; Jang, 2016; Shad et al., 2016). This is called sarcopenia, but it has no single cause. The following is an graph from McLeod et al., 2016 and it shows muscle CSA and how it relates to age:
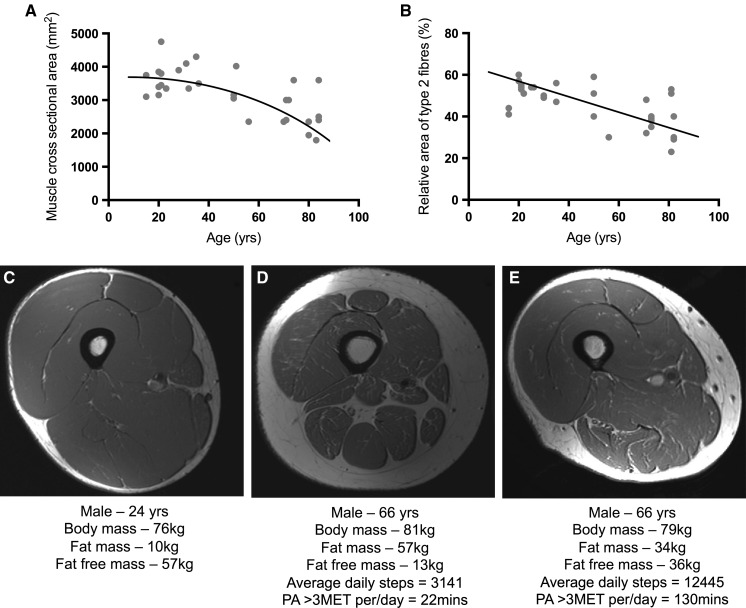
We see a clear downwards trend. In addition to this, Mitchell et al. (2012) suggest that we lose ~5% muscle mass per decade, starting somewhere in our late twenties. Tan et al. (2012) report that skeletal muscle mass drops from ~45% total body weight in our late twenties to ~27% at 70 years old. This result is pretty similar to Mitchell's. Other research groups list more dramatic numbers. They say we could lose 0.5-2% muscle mass per year, starting somewhere in our thirties/early forties 1 2 3. One study actually reports that men observed from 15-83 started atrophying after the age of 25 (McLeod et al., 2016)!!! Looking at these studies together, I think it's safe to assume that people could lose muscle mass in their thirties. However, there are notable individual exceptions (Clark and Manini, 2012), as we will see later when we discuss genetic predispositions.
1: muscle mass fairly consistently decreases at a rate of approximately 0.5–1%/year beginning at 40 years of age (Wagatsuma and Sakuma, 2014).
2: Skeletal muscle mass and strength will gradually decrease each year. For example, about 1 to 2% of skeletal muscle mass declines each year after the age of 30 years [4]. It has been reported that the percentage of muscle mass degeneration is higher in men than in women, at a rate of decline of around 12.9% and 5.3%, respectively, for each decade (Khor et al., 2014)
3: Age-related sarcopenia begins in approximately the fifth decade of life and proceeds, at a population level, at a rate of ∼0.8% annually (Philips, 2015)
In addition to muscle atrophy, we lose strength as we age. Below is an illustration from Delmonico et al., 2009. On the left graph you can see that people that lost muscle mass with aging also got much weaker. On the right side, the subjects gained muscle mass, but still lost strength.

So researchers now believe that there are other mechanisms that impact strength beyond muscle mass. These mechanisms become downregulated with age so that even if you maintain or increase muscle mass, you're bound to get weaker (Mitchell et al., 2012; Clark and Manini, 2012; Wagatsuma and Sakuma, 2014). Several research teams estimate that we lose ~2-4% strength per year as we get older 4 5 6. Note: this number may not directly apply to you if you're actively doing strength training. We will discuss this in more detail further down.
4: Strength decreases with advancing age. Average rates of loss are 2–4% per year. This is 2–5 times faster than muscle mass is lost (Mitchell et al., 2012).
5: Besides the decline in muscle mass, loss of muscle strength is also obvious. Longitudinal studies showed that older adults lose their leg strength around 10 to 15% each decade before their age reaches 70 years, and a more dramatic loss was observed in advanced age, in a range of 2 to 4% per year [5, 6]. Furthermore the declined muscle strength is more severe than the progressive shrink of muscle mass [6, 7]. (Khor et al., 2014)
6: In the fifth decade of life (...) Declines in skeletal muscle strength with sarcopenia, known as dynapenia, are more precipitous at ∼2–3% annually (Philips, 2015)
Researchers now think sarcopenia comes from a combination of many factors, like: muscle disuse, neuron atrophy, CNS inefficiency, declining anabolic hormones, low protein intakes, anabolic resistance, micronutrient deficiency, genetic predisposition, diseases, mitochondrial inefficiency, decreasing muscle quality, and chronic inflammation. I'm going to discuss these mechanisms in the following section.
What causes muscle loss?
Inactivity, atrophy, and muscle quality
As we get older, we're much more likely to be sedentary. Many researchers wonder whether we become sedentary before we lose muscle mass, or whether we become sedentary because we lose muscle mass 7 (Peterson and Gordon, 2011). It's hard to say for sure, but some researchers think muscle mass decreases because muscles lose their quality. By quality I mean ability to produce force. For example, muscles generate less force per unit of CSA with age (Mitchell et al., 2012; McGregor et al., 2014). More fat is deposited into muscle tissue possibly making muscles more inefficient (McGregor et al., 2014; Delmonico et al., 2009; Wagatsuma and Sakuma, 2014). Looking at molecular mechanisms, some studies suggest aging muscles develop problems with muscular contractions because of an impaired calcium release mechanism 8.
7: it is seemingly logical to presume that sarcopenia precedes functional deficit and disability, but it also is likely that disuse itself may lead to exaggerated weakness and thus sarcopenia. (Peterson and Gordon, 2011)
8: A likely muscular contributor to dynapenia is impairment in the excitation–contraction coupling processes, which are series of biophysical events involved in converting the electrical signal for muscle activation into contractile force. Theoretically speaking, the disruption of any of the events in the excitation–contraction coupling process could result in the suboptimal activation of muscle, thus decreasing muscle quality (force per unit tissue area), and contribute to dynapenia. In particular, impairments in calcium (Ca2+) release from the sarcoplasmic reticulum have been suggested to explain the deficits of muscle quality (the intrinsic force-generating capacity of skeletal muscle relative to its tissue size) in aged muscle [113-123]. (Clark and Manini, 2012)
Anabolic resistance
Similarly to muscle quality, anabolic resistance is also a local mechanism. When we look at how the body responds to exercise, we see that it adapts by making our muscles more resistant to change. This has been studied in young lifters and it's called anabolic blunting (Coffey et al., 2005; Mangine et al., 2015; Gonzalez, 2015; Gonzalez et al., 2015a; Noguiera et al., 2015). In short, this blunting means that muscle protein synthesis and mTOR become harder to activate the more trained you get. In theory, this would partially explain why we experience diminishing returns as we grow bigger and stronger. So how does this link to aging? There’s now a debate whether anabolic resistance could happen to older people as well, even if they’re untrained. Some studies find that older lifters show anabolic resistance to strength training and nutrition (Kumar et al., 2009; Vingren et al., 2010; Horstman et al., 2012; Markofski et al., 2015; Moore et al., 2015; Wall et al., 2015; Moro et al., 2016; Timmons & Gallagher, 2016; Loenneke et al., 2016; McLeod et al., 2016; Mitchell et al., 2016; Shad et al., 2016). The theory is that the body slowly downregulates MPS and mTOR in a response to aging.
On the other hand, a new systematic review has just been published and it finds only partial support for the claim that MPS signalling becomes weaker with age (Shad et al., 2016). Shad et al. think a big reason there’s so much contradiction in the MPS literature is because of methodological differences between studies. A big limitation to Shad's review is that the majority of the studies they included only measured mixed muscle protein synthesis. This type of MPS does not predict hypertrophy. We need to look at myofibrillar protein synthesis (myoMPS) (Moore et al., 2009) if we want a shot at predicting gains (and in many cases, myoMPS does not predict gains either) (ASM). Here's an illustration of the differences between mixed MPS and myoMPS post-exercise: (illustration from Damas et al., 2015).
Ideally, Shad et al. would discard studies that measured mixed MPS and only analyze myoMPS. And since Shad isn't here right now I'll do it myself; out of the 24 studies included in Shad's review, 5 dealt with myoMPS. Three studies found a clear difference between young and old in terms of myoMPS responses (Babraj et al., 2005; Cuthbertson et al., 2005; Kumar et al. 2012), one study found differences at some time-points (Kumar et al. 2009), while one study found no difference (Atherton et al., 2016).
Here's an illustration of the differences, as per Kumar et al., 2009:
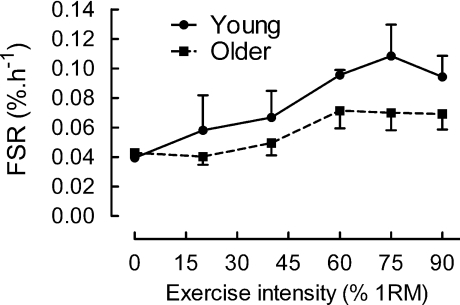
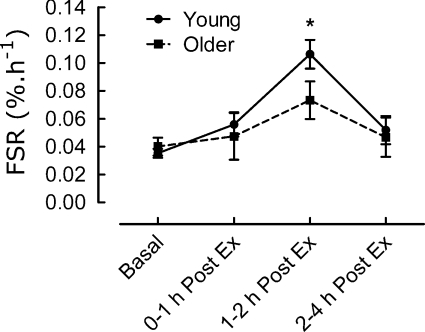
When we eliminate the mixed MPS studies, we see greater support for the anabolic resistance theory. There are also other studies outside of Shad et al's. review that support this (Welle et al., 1993; Welle et al., 1995; Yang et al., 2012). Several of these studies show that mixed MPS is actually quite similar between old and young, but myoMPS is blunted in the old 9 10.
9: Whole body protein synthesis, assessed as the difference between leucine disappearance rate and leucine oxidation, was marginally slower (8%, P = 0.10) in the older group, but not when the data were adjusted for lean body mass. Myofibrillar protein synthesis was a smaller fraction of whole body protein synthesis in the older group (12 vs. 19%). Reduced myofibrillar protein synthesis may be an important mechanism of the muscle atrophy associated with aging. (Welle et al., 1993)
10: Posttraining myofibrillar synthesis was determined on the day after the final training session. There was not a significant change in fractional myofibrillar synthesis in either the young or the old group after training, and the rate in the older group remained 27% slower (P < 0.05). Whole body protein turnover increased approximately 10% only in the younger group, and 24-h urinary 3-methylhistidine excretion (an index of myofibrillar proteolysis) was not significantly affected by training. These data suggest that the slower myofibrillar synthesis rate in older subjects cannot be explained by disuse (Welle et al., 1995)
You might come across studies entitled "Aging does not impair the anabolic response to a protein-rich meal" (Symons et al., 2007), and they might seem convincing at first sight, but once you read conclusions like "Mixed-muscle FSR increased by approximately 51% in both [old and young]" you get disappointed at the researchers for not controlling for myofibrillar MPS.
From the information I've presented here, I think it's likely that MPS-related anabolic resistance exists. Beyond the studies that I've explicitly linked in this section, many of the studies and reviews I discuss in this article agree that MPS-related anabolic resistance is real. However, I will add the limitation that I have not systematically reviewed the literature, so it is possible that there are studies out there that contradict this hypothesis. In that sense, my conclusions are tentative, pending further evidence (as is everything in science...). Furthermore, do note that MPS correlates with gains only in some situations. In most situations researchers have looked at to date, it does not (ASM).
Declining anabolic hormones
Beyond mechanisms that affect the muscle tissue locally, the body experiences systemic changes with age. Most notably, systemic anabolic hormones like IGF-1, GH, and testosterone are reduced (Ryall et al., 2008; Vingren et al., 2010; Horstman et al., 2012; Tan et al., 2012; Fan et al., 2016; Budui et al., 2015). These reductions probably leads to muscle loss (Horstman et al., 2012; Mouser et al., 2016; Vitale et al., 2016). Testosterone drops by about 1-3% per year, beginning sometime during your thirties 11 12, GH and IGF-1 secretion declines by 14% per decade after the age of 30 13, and tissues become more insulin insensitive (Vitale et al., 2016)
Here's a relevant illustration by Ryall et al., 2008:

11: Beginning around the age of 35–40 years, circulating testosterone concentration levels decrease by approximately 1%–3% per year (19) (Horstman et al., 2012)
12: Serum testosterone levels decline at a rate of about 1% per year from the age of 30–40 years in healthy men [7]. (Vitale et al., 2016)
13: Daily GH production has been reported to decline by 14% per decade after the age of 30 years, with a parallel decline in IGF-1 secretion [57]. This may represent an adaptive phenomenon to extend life span through the reduction of cancer risk. In fact GH and IGF-1 are both potent stimulators of cell proliferation [58]. On the other hand, this decline in GH/IGF-1 system contributes to several detrimental phenotypes of aging. (Vitale et al., 2016)
Inflammation
As we age, our bodies develop constant low-level inflammation (Jensen, 2008; Peterson and Gordon, 2011; Fan et al., 2016). Chronic inflammation likely affects muscle mass and strength negatively 14 15 16 17 18 19 20. There are several causes (Fan et al., 2016):

It gets even worse if you have diabetes, because this condition is characterized by inflammation (Park et al., 2009; Kalyani et al., 2014; Khor et al., 2014; Koster and Schaap, 2015; Jang, 2016; Vitale et al., 2016).
14: in conjunction with chronic inflammation and oxidative stress, age-related apoptotic motor neuron loss is proposed to directly attenuate strength, rate of force development, and muscular power,10 and eventually lead to declines in muscle fiber number and physiological cross-sectional area. (Peterson and Gordon, 2011)
15: There is no simple mechanism to explain aging-associated loss of skeletal muscle. It is important to note that impaired cellular immune function combined with low-grade inflammation represents a continuous impact in aging process [7]. (...) aging is associated with prolonged inflammatory activity that is mainly attributed to progressively worsening muscle weakness (Fan et al., 2016)
16: It has been demonstrated that inflammation, along with oxidative stress, increase with aging both are considered significant contributors to age-related muscle wasting process (21, 22). Hence, some studies reported that high IL6 an C-Reactive Protein (CRP) levels are associated with increased risk of muscle mass and strength loss (23, 24). (Budui et al., 2015)
17: Inflammaging is the chronic low-grade inflammatory state present in the elderly, characterized by increased systemic concentrations of proinflammatory cytokines. It has been shown that inflammaging increases the risk of pathologic conditions and age-related diseases, and that it also has been associated with increased skeletal muscle wasting, strength loss, and functional impairments (Draganidis et al., 2016)
18: Sarcopenia is increasingly recognised as an inflammatory state driven by cytokines and oxidative stress [30]. (Robinson et al., 2012)
19: Changes of muscle constituent are another important cause of sarcopenia which is interrelated with other factors such as food intake, lifestyle, and chronic diseases including diabetes mellitus and cardiovascular diseases [34]. Other factors may include hormonal changes and the presence of proinflammatory cytokines [35, 36]. High level of proinflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor (TNF) has been reported to reduce muscle mass and strength [36]. (Khor et al., 2014)
20: the causes of sarcopenia are multi-factorial and can include disuse, changing endocrine function, chronic diseases, inflammation, insulin resistance, and nutritional deficiencies. (Wakabayashi and Sakuma, 2014)
Nervous system inefficiency and neural activation
The nervous system degenerates with age and it goes through multiple changes. Notably, the PNS loses muscle neurons (Frontera et al., 2011; Clark and Manini, 2012; Tintignac et al., 2015). These neurons are nerve cells that go from the spine to muscles and they control muscle contraction. Many authors now think this loss of neurons partially explain why muscles atrophy and we become weaker as we age (Kwan, 2013; McLeod et al., 2016) 21 22 23. However, others say that this might be the case, but we should wait for stronger evidence before we conclude anything for sure 24 (Manini et al., 2013). On the other hand, Manini's review is from 2013, and newer reviews think there is enough evidence to make conclusions (Sakuma et al., 2015). Beyond the physical decrease in motor neurons, the nervous system generally becomes more inefficient at sending messages. Manini et al. (2013) describe this as "neural noise" (i.e. static noise) which leads to "breakdown in communication between brain and muscle". Aging people could therefore have problems with voluntary neural activation and lowered maximal contraction 25.
From Clark and Manini, 2012: (Dynapenia = "Dynapenia is the age-associated loss of muscle strength that is not caused by neurologic or muscular diseases")

21: Further examples [of muscle weakness] are age-related changes in the nervous system such as the loss of motor neurons, the remodeling of motor units through collateral re-innervation, and the impairment of neuromuscular activation manifested as a decreased maximal firing rate of motor units. (Frontera et al., 2011)
22: older adults possess fewer motor units compared with young adults [33,34]. Similarly, it is plausible that the muscle system’s ability to optimally produce force is impaired in dynapenic individuals, with this deficit in the intrinsic force-generating capacity of muscle (force/unit area) caused by potential changes in the excitation–contraction coupling process. (Clark and Manini, 2012)
23: As sarcopenic patients indeed show an increase in motor unit size (119), motor neuron loss may also contribute to the loss of muscle mass although reinnervation of muscle by the surviving motor neurons may delay this process. In addition to this presynaptic effect, there is plenty of evidence that changes in the function and the metabolism of skeletal muscle contribute to sarcopenia (Figure 7). Thus current evidence strongly indicates that sarcopenia is caused by pathological processes in both pre- and postsynaptic cells. (Tintignac et al., 2015)
24: Although age-related strength loss originates from multiple sources, the literature reviewed here suggests that a significant component is the breakdown in communication between brain and muscle. With aging, the changes in the central and peripheral nervous system may reduce an individual’s ability to activate available musculature. While there is a strong theoretical rationale for connecting brain and muscle, there is a general lack of evidence that shows brain aging is associated with muscle strength impairment in older adults. (Manini et al., 2013)
25: The impairments found in central activation are large enough to explain a large portion of observed muscle weakness in a given individual, such as inactivation on the level of ~ 15% or more [50,52,60]. One study that deserves particular attention is by Harridge et al. [50], which entails, to our knowledge, the oldest known cohort of individuals to date to undergo these types of assessments (n = 11, age range 85–97 y). In this study, all older adults required some degree of assistance with everyday activities, and—interestingly—all subjects showed evidence of incomplete voluntary activation during a maximal contraction, with activation ranging from 69% to 93% (mean 81 ± 7%). This finding suggests that deficits in voluntary activation can contribute to a significant portion of the muscle weakness observed in the very old. (...) Collectively, these findings suggest that aging results in decreased motor cortical excitability and cortical plasticity, which may contribute to age-related decreases in muscle performance. (Clark and Manini, 2012)
Genetics
Genetics is a huge and complicated subject so I will try to simplify as much as possible here. Researchers think people have different phenotypes. A phenotype is basically a collection of observable traits (physical features, mental abilities, etc.). Phenotypes are created from an organism's genes and their interaction with the environment. We now think that some phenotypes are at greater risk for sarcopenia because of heritability. It's estimated that muscle strength is 30-85% genetically inherited, while muscle mass is 45-90% inherited (Roth, 2012; Pereira et al., 2013), but the estimations vary depending on which review and study we look at (Tan et al., 2012). Update: a new systematic review and meta-analysis suggests strength is about 50% inherited (Zempo et al., 2016).
In addition to heritability, we have individual variation. Some individuals could lose a lot of muscle mass as they age, while others do not (Clark and Manini, 2012; Tan et al., 2012). This has been studied recently, and some older people that do strength training actually lose muscle mass. These people are referred to as non-responders, and it also happens in young subjects. You can see the non-responders at the bottom of this graph by Churchward-Venne et al., 2015:

There are some issues with measuring fat mass, LBM, water, etc. using DXA/BIA and similar methods. However, I won't go into that topic in this review.
We can also look at individual genes and analyse their relationship to muscle loss. The ACE, ACTN3, MSTN (myostatin), CNTF and VDR (vitamin D) genes are promising 26 (Khor et al., 2014). Some authors claim each of these genes can contribute 1-3% when it comes to skeletal muscle variation 27. It's possible that genes can also have synergistic effects. For example some genes could have a much stronger effect when combined 27. In that sense, no gene exists in isolation.
26: The ACE, ACTN3, MSTN, CNTF and VDR genes have been associated with skeletal strength and/or mass in two or more studies. The MSTN gene was a strong contributor to variation in skeletal muscle phenotypes supported by the concordance of linkage studies, association studies and expression studies. (Tan et al., 2012).
27: Of those genes that have been identified, their importance to skeletal muscle-trait variation is generally small. None of the genes described above have been shown to conclusively contribute >5% of the inter-individual variation to their respective traits, and most are on the order of 1–3%. In addition to typical polymorphisms, copy number variation (multiple copies of the same gene), gene–gene interactions (multiple genes coordinated in a pathway), complex gene–environment interactions and epigenetic factors also contribute to the genetic component of inter-individual variability (Roth, 2012)
Low protein intakes
It's highly likely that sarcopenia is affected by low protein intakes (Shad et al., 2016). In fact, we need more protein the older we get (Moore et al., 2015; Philips, 2015; Shad et al., 2016; Mitchell et al., 2016; McLeod et al., 2016; Loenneke et al., 2016; Courtney-Martin et al., 2016; Baum et al., 2016). Adults that eat a lot of protein maintain ~40 % more muscle mass compared to those that eat very little protein 28.
[themify_icon icon="fa-quote-left"] 28: data from the Health ABC study highlighted that older individuals in the highest quintile for protein intake (~19 % of total energy intake) lost ~40 % less lean mass than did those in the lowest quintile for protein intake (~11 % of total energy intake) (McLeod et al., 2016)
Micronutrient deficiency
Micronutrients are vitamins and minerals. It generally goes without saying that it's really important to get enough micros regardless of age. There are some micros that are particularly interesting; vitamin D. In general, low vitamin D levels could lead to muscle atrophy and reduced strength (Robinson et al., 2012; Roth, 2012; Wagatsuma and Sakuma, 2014; Khor et al., 2014; Wakabayashi and Sakuma, 2014; Budui et al., 2015). Nevertheless, there's a big BUT here: pretty much every review of the vitamin D literature agrees that results are inconclusive, so we can't say for sure how much vitamin D deficiency matters when it comes to strength and muscle mass.
Other causes
I don't have the time nor inclination to look at every single potential cause for sarcopenia in this article, but I will link you an illustration from (Miljkovic et al., 2015) that summarizes some potential causes:

How to treat and prevent age-related muscle loss
The following section is presented to you for information purposes only. It is not medical advice. Please consult your doctor before you consider any drastic measures.
Protein recommendations
The single most important recommendation from pretty much every review I've read on the issue is to increase your protein intake (Shad et al., 2016; Mitchell et al., 2016; McLeod et al., 2016). These are the recommendations from recent reviews: eat 30 to 50 grams protein per meal (Philips, 2015; Loenneke et al., 2016; Shad et al., 2016) and try to get at least 1.2g protein per kg bodyweight throughout the day (Shad et al., 2016; McLeod et al., 2016; Courtney-Martin et al., 2016). However, a recent review by Baum et al., 2016 criticizes these recommendations because they do not account for factors like muscle protein breakdown. Baum et al., think these recommendations are still too mild, and that older adults need to up protein intake to ~35% of their total caloric intake. So let's do some math:
Let's say you have a 60 year old man who weights 70 kg. His TDEE is 2200 per day. According to the "mild" recommendations, he needs at least 70kg*1.2g = 84grams of protein per day. 84g*4 = 336 kcal. 336kcal/2200kcal = 15% protein of daily caloric intake. Now if we use Baum's model, the man would have to double his protein consumption to 168g. However, Baum et al. admit that "practical limitations may make this level of dietary protein intake difficult" (Baum et al., 2016).
Recommendation: It's hard to say exactly what the recommendation should be, but I think it's fair to suggest 1.2 protein per kg bw is the lowest acceptable limit, with protein intakes up to and (possibly) exceeding 2g per kg bw, especially if you're doing resistance training. Please consult your doctor before you drastically increase protein intake (in case you have kidney issues that could be exasperated with increased protein load) (Examine.com)
Some studies show that protein supplementation could have an anti-inflammatory effect in addition to being anabolic (Draganidis et al., 2016).
Resistance training
Strength training, along with high protein intakes, prevents muscle atrophy and it becomes more important with age (Hurley and Roth, 2000; Roth et al., 2000; Martel et al., 2006; Jensen, 2008; Peterson et al., 2010; Peterson and Gordon, 2011; Basharat et al., 2012; Mitchell et al., 2012; Sakuma et al., 2014; Wakabayashi and Sakuma, 2014; Philips, 2015; Sakuma et al., 2015). One meta-analysis suggests higher intensity improves strength to a greater extent in older adults (Peterson et al., 2010). A follow-up review by the same authors reported that higher volumes are better for hypertrophy in aging adults (Peterson and Gordon, 2011). This is now affirmed by a recent meta-analysis by Schoenfeld et al. (2016). Here are Schoenfeld's recommendations:
Based on our findings, it would appear that performance of at least 10 weekly sets per muscle group is necessary to maximise increases in muscle mass. Although there is certainly a threshold for volume beyond which hypertrophic adaptations plateau and perhaps even regress due to overtraining (...) the optimal RT dose will ultimately vary between individuals, and these differences may have a genetic component (...) practitioners should carefully monitor client progression and adjust training dosages based on the individual’ s response. (Schoenfeld et al., 2016)
From a molecular perspective, doubling volume from 3 to 6 sets enhances MPS in older men but not young men (Kumar et al., 2012). Like I've said before, it's difficult to predict gains with MPS, but I still wonder why MPS continues to increase in older men while it stops "early" in young men.
Hormone therapy
I am not making any recommendations in this section. It is provided for informational purposes only.
Low testosterone levels might lead to loss of muscle mass, declining strength, lowered bone mass, and more central body fat (Horstman et al., 2012; Mouser et al., 2016). Some authors suggest testosterone replacement therapy as a solution (Khor et al., 2014; Vitale et al., 2016). However, they emphasize that it has some health risk, especially if you take testosterone doses that are supra-physiological (beyond natural ranges) (Horstman et al., 2012; Khor et al., 2014; Budui et al., 2015; Vitale et al., 2016).
Lifestyle
One study found that making young men walk less gave them temporary anabolic resistance (i.e. blunted MPS response to protein) (Moro et al., 2016). There's reason to believe that an active lifestyle (outside of the gym) is generally better for maintaining muscle mass and physical function (Malafarina et al., 2013; Atkins et al., 2014). For example, frequent exercise could reduce systemic inflammation (Teeman et al., 2016)
Preventing chronic inflammation through supplementation
As mentioned, chronic inflammation could cause atrophy (Horstman et al., 2012). There are some ways to fight this inflammation:
- Vitamin E (Khor et al., 2014; Rondanelli et al., 2015)
- Omega-3s (Smith, 2016)
- Protein supplementation (Draganidis et al., 2016).
- Avoiding and/or treating diabetes
- Vitamin D (might be anabolic) (Robinson et al., 2012; Wagatsuma and Sakuma, 2014; Morley, 2016)
However, I should note that there are a lot of studies that show supplementing large doses of antioxidants (vitamin A, C, E) can actually prevent training adaptations in young subjects. So be careful about micronutrient supplementation if you're not deficient. I will publish an article on this in the future.
Conclusions
We lose muscle mass and become weaker as we age. This is due to various neurological, muscular, hormonal, and molecular mechanisms that change as we age. Our nervous system becomes more inefficient, our muscle quality decreases, our anabolic hormone secretion decreases, our bodies develop chronic inflammation, and we develop anabolic resistance. There's still a lot of discussion as to which of these mechanisms is the primary cause of muscle loss. Regardless of which one is the "primary cause", most authors agree that we start to lose muscle mass during our 30s and that there are many small causes rather than one big one. In any event, we can prevent muscle loss by ingesting more protein (at least 1.2g/kg bw), by strength training with high intensities and solid volumes, by having an active lifestyle outside the gym, and by supplementing various nutrients like vitamin E, D, and omega-3s (if you are deficient). There are also riskier solutions, but for those you have to see your doctor because I cannot give you medical advice.
Our genetics also influence how susceptible we are to muscle loss as we age. Some people are simply more likely to win the genetic lottery, even though I know saying this has a strong undertone of eugenics. But I promise that insurance companies won't charge you more for your "bad genes" that predispose you to disease and sarcopenia... Yet...
[expand title="Limitations"]
Given that this is not a peer-reviewed systematic review or meta-analysis, this article is inherently at risk of study selection bias. I always write these reviews/opinions in the state of mind of an open-minded learner, but I can’t exclude the possibility of bias when selecting, interpreting, or discussing these studies. In fact, I would argue that I most likely am biased, as we all are. Furthermore, I do not have the time to represent the literature in its entirety nor evaluate the quality of every study in detail. This means that some of the studies I discuss or the studies they reference may be found to be flawed if examined in detail. If this is the case, it follows that my conclusions using these studies as premises would be flawed. However, the power of this article is not its qualitative analysis of every study; it is that it represents many of the major and most important publications on anabolic signaling mechanisms as well as the authors’ discussions of these studies.[/expand]